SIM Automation GmbH, Germany


Company: SIM Automation GmbH
Industry: Medical Technology
DENSO Products Used: HSR-065-N32
Company location: Germany
Website: www.sim-automation.de
CHALLLENGE
The medical technology supply industry has been facing huge issues not only since the Covid-19 pandemic: On the one hand, conventional production for laboratory needs could not keep up due to the surging increase in demand – from test vials to test tubes and test kits; on the other hand, the sector was already exposed to cost pressure due to increasing global competition. Faced with two challenges at the same time, manufacturers around the world are responding with automating their production. According to current estimates, the market volume for robots used in med-tech will more than double by 2025 (compared to 2020)1 , with the segment for robot-assisted production of medical consumables making up the largest growth of with 18.5 % annually.2
This includes, for example, a small but indispensable product for any hospital or laboratory – test vials filled with reagents (reactants) for blood tests, which not only have to be manufactured and filled in huge quantities, but also mounted in a transport lock so that they are not moved too much or even damaged in the logistics chain. In addition, strict production regulations and documentation requirements in the pharmaceutical and medical sectors make it difficult to mass-produce delicate products such as these; a major challenge for manual assembly.
1 https://www.marketsandmarkets.com/Market-Reports/medical-robotic-systems-market-2916860.html
2 https://www.marketsandmarkets.com/Market-Reports/medical-robotic-systems-market-2916860.html
THE SOLUTION
An automated, robotic assembly system from SIM Automation offers the optimal approach here. For more than 60 years, the company has been shaping the market for turnkey systems with its automation solutions, which are primarily designed for high output of micro-precision parts and equipped with state-of-the-art software. SIM Automation, based in Heilbad Heiligenstadt near Göttingen, Germany, has 200 employees with more than 2,700 systems installed worldwide to date.
With medical technology systems, the focus is on compliance with the required cycle times and the absence of errors during sorting, loading and filling of the components. Accordingly, assembly processes with millimeter precision and high cycle performance are used, controlled by user-defined software in accordance with the latest programming standards as per DIN EN 61131.
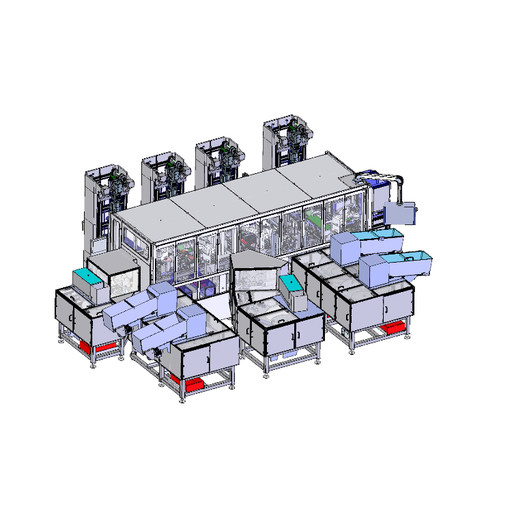
The assembly system developed by SIM Automation for a medical technology manufacturer assembles and palletizes small plastic vials, each with volumes between 5 and 60 ml for holding reagents required, among other things, for measuring the PH value in blood tests. Four robots from DENSO Robotics' innovative HSR series operate at the center of the system: the high-performance SCARA series features three models with different arm lengths (480, 550 and 650 mm) and a payload capacity of up to 8 kg.
In the three-tier system, the four SCARA HSR-065-N32 robots operate directly next to each other on an automated process line: the robots work closely together by placing and assembling each container in a vial holder – a pick-and-place task that seems straightforward at first glance, but proves to be challenging due to the high cycle rate, the required precision and the coordinated process flow. The vials are provided in a pallet on the side of the robot, which then picks them individually before placing them.
The SCARA robots are controlled via DENSOs compact RC8A controllers, which control the high speed and high accurate robot movements. Also connected to the controllers is the Teach Panel, which allows programming, operation, teach-in as well as maintenance of the robots via a user-friendly touch screen. The interface to the PLC control of the entire plant runs via PROFINET.
SIM Automation's decision for DENSO Robotics was an obvious choice, as the company has been using various DENSO robots (SCARA models as well as 6-axis models) in a wide variety of systems for years – they can be integrated easily and flexibly according to the specific application and particularly high reliability and long lifetime. And the four-axis SCARA robots are designed precisely for high-performance, automated applications such as this one –with cycle times between 0.28 and 0.31 sec and a repeatability of ±0.01 mm to ±0.012 mm. The robots can therefore implement a very high number of cycles per minute, while responding promptly in industrial applications, operating continuously at optimum speed, and still complete each operation precisely.
As the facility relies on the interaction of the devices, their integration plays an important role. In order to maintain a high level of flexibility in the overall system, a service-based software architecture was implemented. An adaptive, specially designed software system controls all processes. It can respond flexibly to indicators for cell culture such as cell growth and, if necessary, execute individual services following a modular approach. These services are provided by all devices via an Integration Framework, which allows the user to create any combination of services at the control level. Each device offers services with defined parameters that can be controlled manually via the intuitive user desktop or assembled into complex combinations. In- and outgoing data collected from the devices are provided in a universal format so users can control all devices from a single desktop – whether it is the microscope or the DENSO robot, the liquid handling unit or the incubator and repositories. The software has been programmed in C#, which makes it possible to abstract complex logical relationships and implement object-oriented programming.
ADVANTAGES
The advantages of an automated system are obvious: the increase in efficiency alone compared to manual assembly is considerable: the system assembles 72 vials per minute, i.e. 4,320 plastic containers in one hour – by comparison, manual assembly would take around six hours for the same number (calculated on the basis of 5 seconds processing time per container).
But the decisive advantage of automation in this case is product quality: For medical technology, this is crucial; after all, these are health-relevant products where a continuous, reliable safety is a must. It’s guaranteed not only by the automation itself, but also by the technical quality control. In addition, seamless data tracking all the way to the end product significantly optimizes product safety. At the same time, all GMP (Good Manufacturing Practice) regulations – the legal minimum requirements for manufacturing practice stipulated by the European Medicines Agency (EMA) for medical technology production – are met in full.